Which of the following is an exergonic reaction? This question embarks us on a captivating journey into the realm of thermodynamics, where we unravel the mysteries of energy transfer and chemical reactions. Exergonic reactions, characterized by their spontaneous nature and energy release, play a pivotal role in diverse scientific disciplines, from biology to chemistry and physics.
Delving into the intricacies of exergonic reactions, we will explore the fundamental concepts of enthalpy and entropy, and their profound influence on reaction spontaneity. Through the lens of Gibbs free energy, we will gain insights into the driving forces behind these reactions and their significance in biological systems and practical applications.
Define Exergonic Reactions

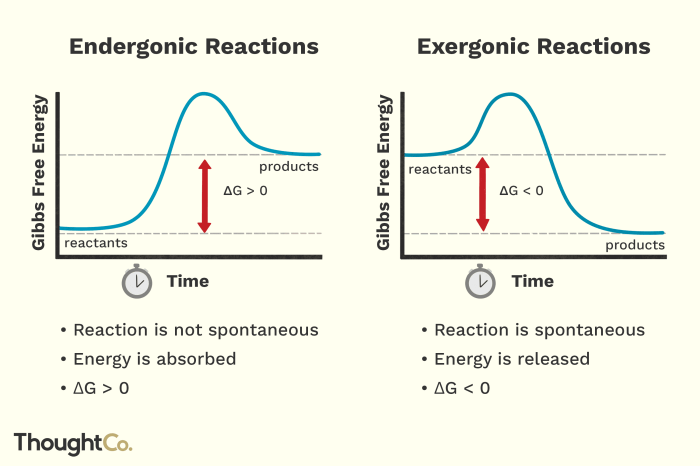
Exergonic reactions are chemical reactions that release energy in the form of heat, light, or electrical energy. These reactions are characterized by a negative change in free energy, indicating that the products of the reaction are more stable than the reactants.
Exergonic reactions are often spontaneous and occur without the need for an external energy input. This spontaneity is due to the decrease in free energy, which drives the reaction towards completion.
Examples of Exergonic Reactions
- Cellular respiration
- Neutralization reactions
- Exothermic reactions
Thermodynamics of Exergonic Reactions
The thermodynamics of exergonic reactions can be explained using the concepts of enthalpy and entropy.
Enthalpy (H) is a measure of the total energy of a system, including both potential and kinetic energy. Entropy (S) is a measure of the disorder or randomness of a system.
In exergonic reactions, the enthalpy change (ΔH) is negative, indicating that the products have less energy than the reactants. The entropy change (ΔS) can be either positive or negative, depending on the reaction.
The spontaneity of an exergonic reaction is determined by the Gibbs free energy change (ΔG), which is calculated using the following equation:
ΔG = ΔH – TΔS
where T is the temperature in Kelvin.
If ΔG is negative, the reaction is spontaneous. If ΔG is positive, the reaction is non-spontaneous and requires an external energy input to proceed.
Examples of Exergonic Reactions
Exergonic reactions occur in a wide variety of fields, including biology, chemistry, and physics.
Biology
- Cellular respiration: The breakdown of glucose to produce energy
- Muscle contraction: The release of energy from ATP
- Photosynthesis: The conversion of light energy into chemical energy
Chemistry
- Neutralization reactions: The reaction between an acid and a base to form a salt and water
- Combustion reactions: The reaction between a fuel and oxygen to produce heat and light
- Electrochemical reactions: The conversion of chemical energy into electrical energy
Physics, Which of the following is an exergonic reaction
- Radioactive decay: The release of energy from the nucleus of an atom
- Nuclear fusion: The combination of two atomic nuclei to form a heavier nucleus and release energy
- Nuclear fission: The splitting of an atomic nucleus into two or more smaller nuclei and release energy
Importance of Exergonic Reactions
Exergonic reactions are essential for life on Earth.
In biological systems, exergonic reactions provide the energy needed for cells to function. These reactions are responsible for energy production, metabolism, and other cellular processes.
Exergonic reactions are also important in the environment. For example, the combustion of fossil fuels releases energy that is used to power cars, generate electricity, and heat homes.
Applications of Exergonic Reactions: Which Of The Following Is An Exergonic Reaction
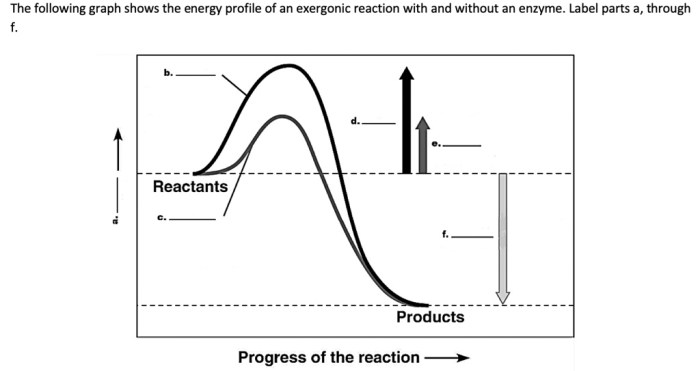
Exergonic reactions are used in a wide variety of practical applications.
- Energy generation: Exergonic reactions are used to generate electricity in power plants and batteries.
- Food preservation: Exergonic reactions are used to preserve food by killing bacteria and preventing spoilage.
- Industrial processes: Exergonic reactions are used in a variety of industrial processes, such as the production of chemicals, plastics, and metals.
FAQ Explained
What is the key characteristic of an exergonic reaction?
Exergonic reactions are characterized by a negative change in Gibbs free energy, indicating a spontaneous release of energy.
How does enthalpy contribute to exergonic reactions?
Enthalpy, a measure of heat flow, plays a crucial role in exergonic reactions. A negative change in enthalpy signifies the release of heat, contributing to the overall energy decrease.
What is the significance of exergonic reactions in biological systems?
Exergonic reactions are essential for energy production and metabolism in living organisms. They drive cellular processes such as ATP synthesis and muscle contraction.