Empirical formula and molecular formula worksheet with answers – Welcome to the realm of chemistry, where empirical and molecular formulas play a pivotal role in deciphering the composition of substances. This comprehensive worksheet, complete with detailed solutions, embarks on an educational journey to unravel the mysteries of these fundamental concepts, empowering you with the knowledge to determine the elemental makeup of compounds with precision.
Empirical Formula and Molecular Formula

In chemistry, empirical formulas and molecular formulas are used to represent the composition of compounds. An empirical formula gives the simplest whole-number ratio of the elements present in a compound, while a molecular formula indicates the actual number of atoms of each element in a molecule of the compound.
Empirical Formula
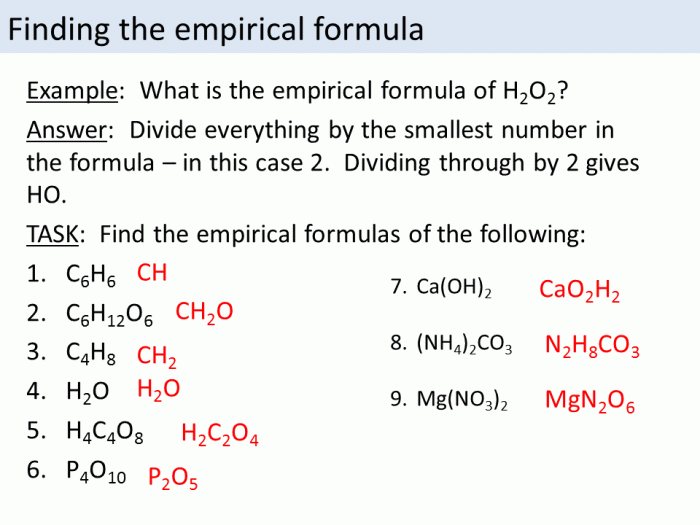
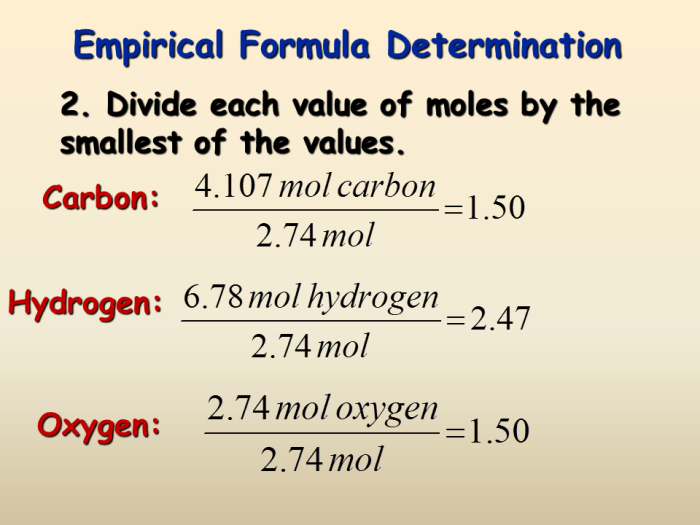
An empirical formula is the simplest whole-number ratio of the elements present in a compound. It is determined by finding the mass of each element in the compound and dividing by the atomic mass of the element. The resulting numbers are then converted to whole numbers by dividing by the smallest number.
For example, if a compound is found to contain 12.01 g of carbon, 2.016 g of hydrogen, and 32.00 g of oxygen, the empirical formula is CH 2O.
Molecular Formula
A molecular formula indicates the actual number of atoms of each element in a molecule of a compound. It is determined by finding the empirical formula of the compound and then multiplying the subscripts in the empirical formula by a whole number that gives the correct molecular mass.
For example, the empirical formula of glucose is CH 2O. The molecular mass of glucose is 180.15 g/mol. To find the molecular formula, we divide the molecular mass by the empirical formula mass (30.03 g/mol) and get 6. This means that the molecular formula of glucose is C 6H 12O 6.
Worksheet with Answers, Empirical formula and molecular formula worksheet with answers
A worksheet with practice problems on determining empirical and molecular formulas is provided below.
| Problem | Solution |
|---|---|
| A compound is found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen. What is the empirical formula of the compound? | CH2O |
| A compound has an empirical formula of CH2O and a molecular mass of 180.15 g/mol. What is the molecular formula of the compound? | C6H12O6 |
Additional Resources
- Empirical and Molecular Formulas
- How to Solve Empirical and Molecular Formula Problems
- Empirical and Molecular Formulas: What’s the Difference?
Questions and Answers: Empirical Formula And Molecular Formula Worksheet With Answers
What is the difference between empirical and molecular formulas?
Empirical formulas represent the simplest whole-number ratio of elements in a compound, while molecular formulas indicate the actual number of atoms of each element present in a molecule.
How do I determine the empirical formula from experimental data?
Divide the mass of each element by its respective atomic mass and find the simplest whole-number ratio.
What methods can I use to determine the molecular formula from the empirical formula?
Multiply the empirical formula by an appropriate factor to match the molecular weight.