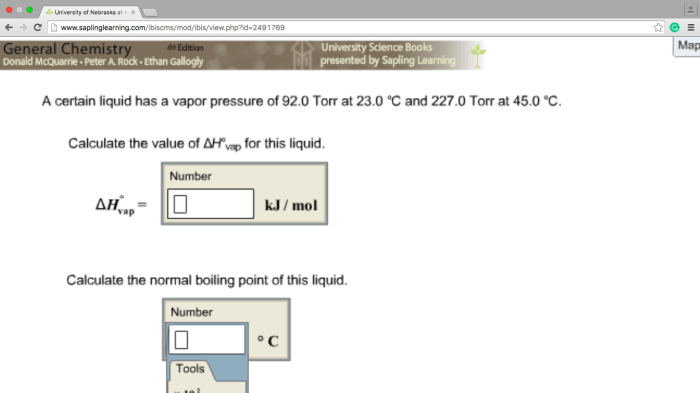
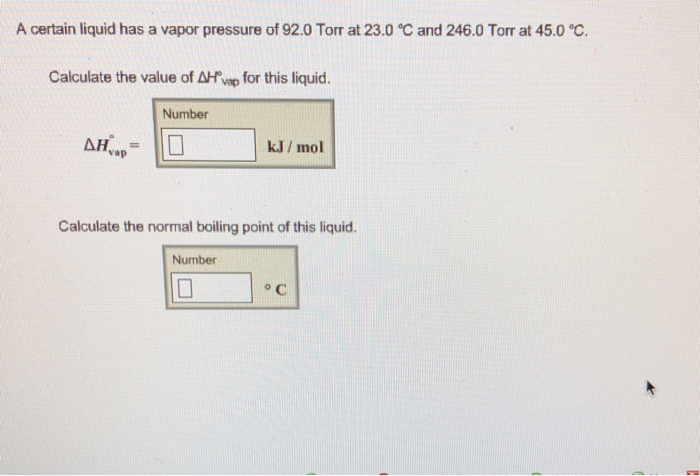
A certain liquid has a vapor pressure of 92.0 torr – In the realm of chemistry, a certain liquid has captured our attention with its intriguing vapor pressure of 92.0 torr. This value holds profound significance in understanding the liquid’s behavior and its interactions with its surroundings. As we delve into this topic, we will explore the fundamental concepts of vapor pressure, its influencing factors, measurement techniques, and its diverse applications across various fields.
Vapor pressure plays a pivotal role in determining the volatility and evaporation rate of a liquid. It provides valuable insights into the intermolecular forces that govern the liquid’s behavior. By examining the vapor pressure of a certain liquid, we can gain a deeper understanding of its molecular structure and its suitability for specific applications.
Vapor Pressure Definition and Significance
Vapor pressure refers to the pressure exerted by the vapor of a liquid when it is in equilibrium with its liquid phase. It is a measure of the tendency of a liquid to vaporize and is a key property in understanding the behavior of liquids.
Vapor pressure is crucial in various fields, including chemical engineering, meteorology, and environmental science. It plays a significant role in processes such as evaporation, distillation, and boiling, and helps predict the behavior of liquids in different environments.
Factors Affecting Vapor Pressure
Temperature
Temperature has a direct impact on vapor pressure. As temperature increases, the kinetic energy of liquid molecules increases, leading to a higher number of molecules escaping the liquid and entering the vapor phase, resulting in an increase in vapor pressure.
Molecular Weight
Vapor pressure is inversely proportional to molecular weight. Heavier molecules have weaker intermolecular forces, making it more difficult for them to escape the liquid phase. Consequently, liquids with higher molecular weights tend to have lower vapor pressures.
Intermolecular Forces, A certain liquid has a vapor pressure of 92.0 torr
Intermolecular forces play a crucial role in determining vapor pressure. Liquids with stronger intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, have lower vapor pressures because more energy is required to overcome these forces and escape the liquid phase.
Measuring Vapor Pressure: A Certain Liquid Has A Vapor Pressure Of 92.0 Torr
Manometric Method
The manometric method is a simple and direct approach to measuring vapor pressure. It involves connecting a closed container containing the liquid to a manometer. As the liquid evaporates, the pressure inside the container increases, which is measured by the manometer.
Bubbling Method
The bubbling method measures vapor pressure by bubbling an inert gas through the liquid. The pressure of the gas is gradually increased until bubbles start to form. The vapor pressure of the liquid is then equal to the pressure of the gas at the point of bubbling.
Vapor Pressure and Liquid-Vapor Equilibrium
Liquid-vapor equilibrium occurs when the rate of evaporation equals the rate of condensation. At this point, the vapor pressure of the liquid is constant and is known as the equilibrium vapor pressure.
The boiling point of a liquid is the temperature at which its vapor pressure becomes equal to the external pressure. At this point, the liquid can vaporize throughout its entire volume, resulting in boiling.
Applications of Vapor Pressure
Chemical Engineering
Vapor pressure is crucial in chemical engineering for designing and optimizing processes involving distillation, evaporation, and drying. It helps determine the operating conditions and equipment specifications for these processes.
Meteorology
In meteorology, vapor pressure plays a key role in understanding atmospheric conditions and predicting weather patterns. It is used to calculate relative humidity, which is an important factor in cloud formation and precipitation.
Quick FAQs
What is the significance of vapor pressure?
Vapor pressure is a crucial property that provides insights into a liquid’s volatility, evaporation rate, and intermolecular interactions.
How is vapor pressure measured?
Vapor pressure can be measured using various techniques, including the manometer method, the boiling point method, and the gas chromatography method.
What factors influence vapor pressure?
Vapor pressure is primarily influenced by temperature, molecular weight, and intermolecular forces.
What is the relationship between vapor pressure and boiling point?
Vapor pressure and boiling point are closely related. The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure.