Experiment 9 molar mass of a volatile liquid – Embarking on Experiment 9: Determining the Molar Mass of a Volatile Liquid, we delve into the fascinating realm of chemistry, where we unravel the secrets of molecular structures and their properties. This experiment empowers us to uncover the molar mass of a volatile liquid, a crucial parameter that unveils its identity and behavior.
Volatile liquids, characterized by their tendency to readily vaporize, play a significant role in various scientific and industrial applications. Understanding their molar mass is paramount, as it governs their physical and chemical properties, influencing their reactivity, solubility, and other key characteristics.
Introduction: Experiment 9 Molar Mass Of A Volatile Liquid
Molar mass is a fundamental property of a substance that represents its mass per mole. It is a critical parameter in chemistry, enabling the determination of the number of molecules or formula units present in a given mass of a substance.
In this experiment, we aim to determine the molar mass of a volatile liquid, a substance that readily vaporizes at room temperature.
Materials and Equipment
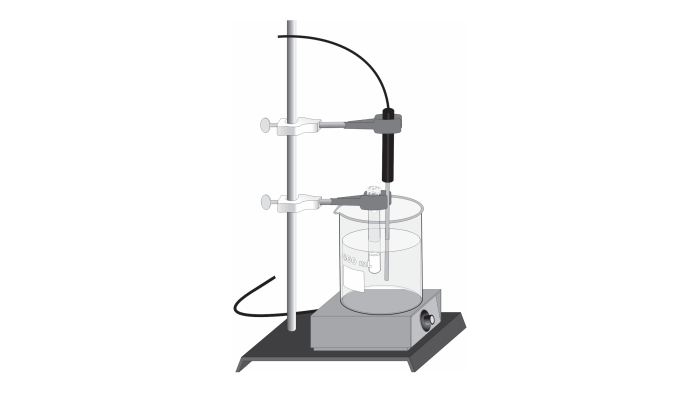
- Volatile liquid (e.g., acetone, ethanol)
- Analytical balance
- Gas syringe
- Thermometer
- Barometer
- Safety goggles
- Gloves
Experimental Procedure
- Measure the mass of an empty gas syringe.
- Inject a small volume of the volatile liquid into the syringe.
- Record the temperature and atmospheric pressure.
- Heat the syringe gently to vaporize the liquid.
- Measure the volume of the vapor at constant temperature and pressure.
- Repeat steps 2-5 for different masses of the volatile liquid.
Data Analysis

The molar mass of the volatile liquid can be calculated using the ideal gas law:
PV = nRT
where:
- P is the pressure (in Pa)
- V is the volume (in m 3)
- n is the number of moles
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature (in K)
Rearranging the ideal gas law, we get:
n = PV/RT
The molar mass (M) can be calculated by dividing the mass of the volatile liquid (m) by the number of moles (n):
M = m/n
Discussion
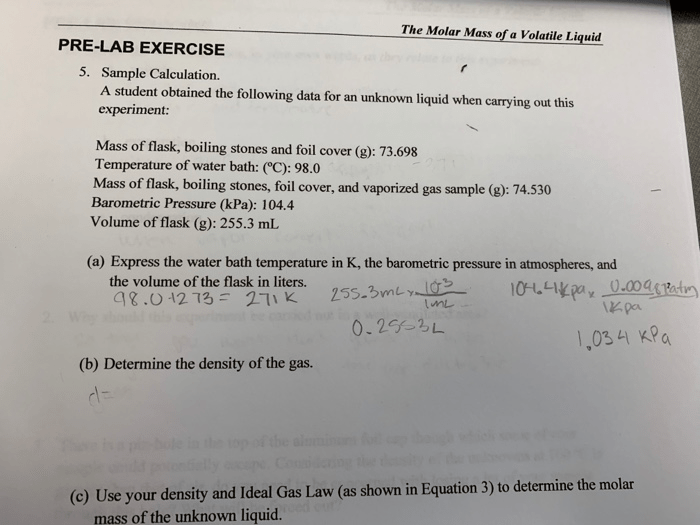
The determined molar mass of the volatile liquid can be compared to its known value or theoretical prediction. Deviations from the expected value may indicate experimental errors or the presence of impurities. The accuracy of the experiment can be improved by using precise measuring equipment and minimizing sources of error, such as temperature fluctuations or leaks in the apparatus.
Questions and Answers
What is the significance of molar mass in chemistry?
Molar mass is a fundamental property that represents the mass of one mole of a substance. It provides insights into the molecular weight and composition of compounds, enabling the determination of their empirical and molecular formulas.
How does the volatility of a liquid affect its molar mass determination?
Volatile liquids readily vaporize, which can pose challenges in accurately measuring their mass. Specialized techniques, such as the vapor density method employed in this experiment, are employed to overcome these challenges and obtain reliable molar mass values.
What are the potential sources of error in this experiment?
Errors can arise from inaccuracies in measuring the mass of the liquid and its vapor, as well as variations in temperature and pressure during the experiment. Careful calibration of equipment and precise experimental techniques are essential to minimize these errors.